
Trinity Biotech (NASDAQ: TRIB) announced today that it secured a massive order for 9 million units of its TrinScreen HIV rapid diagnostic test, which is a World Health Organization-prequalified product used in high-volume HIV screening programs worldwide.
Here’s how the market responded to the news …
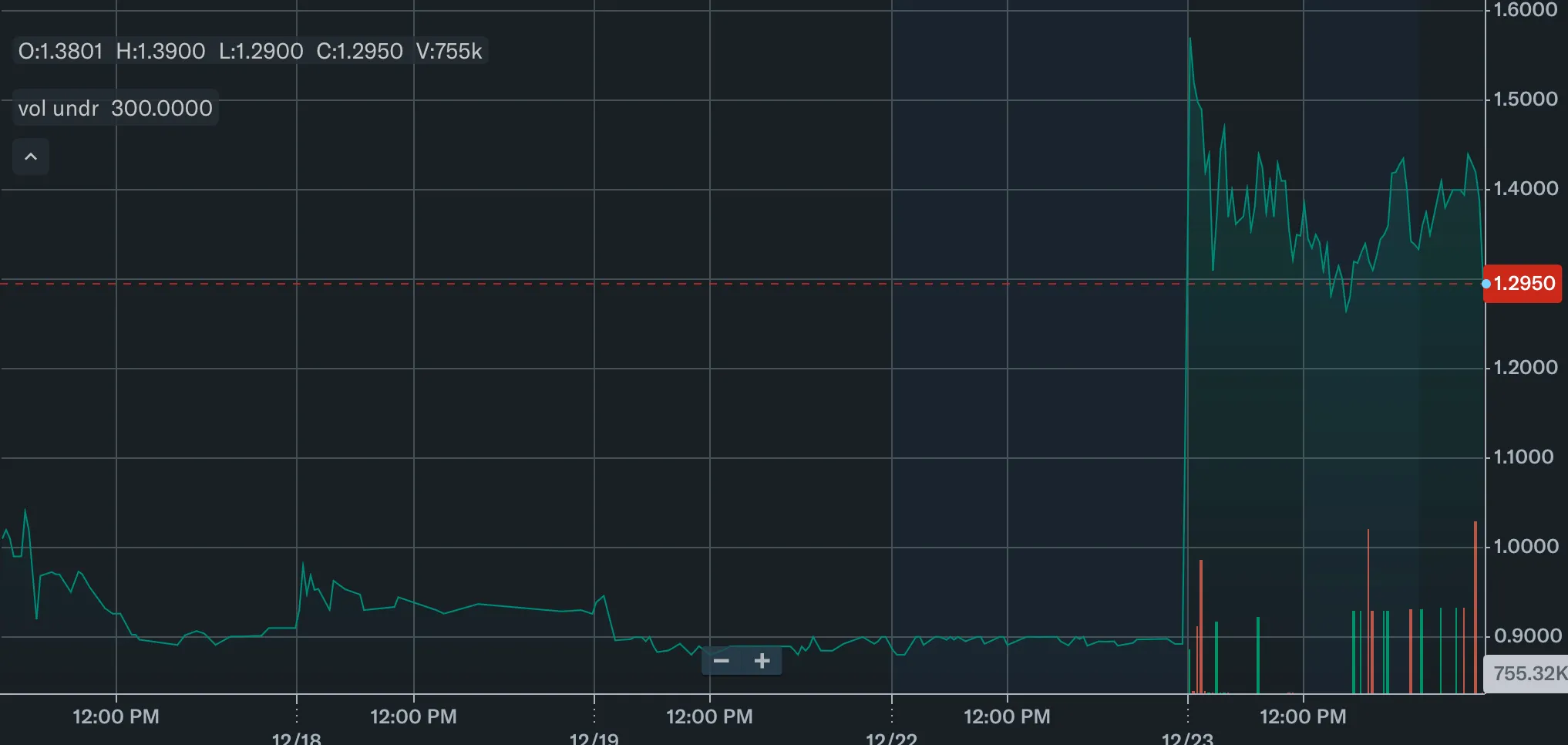
Delivery of the tests is scheduled for Q4 2025 and Q1 2026, reflecting renewed global demand for HIV screening after earlier funding disruptions.
This deal reinforces the company’s position in a market that is not only large but also structurally durable.
To be sure, HIV testing isn’t discretionary. It’s driven by public-health mandates, government funding, NGO procurement, and ongoing global screening programs. This is why today’s move matters.
HIV tests are repeat-use diagnostics. They generate recurring revenue, not one-off sales. And unlike early-stage biotech announcements, this deal isn’t about future possibilities; it’s about products that are already validated, approved, and needed now. That distinction is critical, and the math is simple. A confirmed HIV testing agreement…
- Improves near-term revenue visibility
- Strengthens Trinity’s diagnostics portfolio
- Reinforces the company’s relevance in global health supply chains
In other words, this isn’t a science story; it’s a commercial execution story.
Is this a moonshot?
No.
But markets don’t move on moonshots alone. They move on credibility, contracts, and cash flow potential. And an HIV test deal checks all three boxes, especially in a world where most global health programs remain well-funded and politically insulated.
Trinity didn’t have an easy year, so today’s news was a welcomed surprise for shareholders.








.webp)