
Big news out of the Compass Pathways (NASDAQ: CMPS) camp today.
The company just nailed its second Phase 3 trial for treatment-resistant depression (TRD).
Not Phase 2.
Not “promising early data.”
I’m talking late-stage, pivotal, big-league clinical results.
And when you get two successful Phase 3 trials in a row? That’s when Wall Street starts taking you seriously, especially when it comes to this condition.
If you’re unfamiliar, TRD is a form of major depressive disorder in patients who have not responded to at least two adequate trials of different antidepressant medications or standard treatments.
Truth is, Big Pharma has been trying to crack this for decades. And Compass may have just brought a legitimate treatment closer to fruition.
Here’s what CEO Kabir Nath had to say about the results …
Across three robust, well-designed, and well-executed clinical trials involving more than 1,000 participants, we have now demonstrated consistent, highly statistically significant results at the primary endpoint and a clinically meaningful effect.
This is a remarkable achievement for the field of psychiatry - especially in the TRD population, where proving benefit has historically been extraordinarily challenging. These data strengthen our conviction in the highly differentiated profile for COMP360. Given the urgent need for new treatments in TRD, we are advancing our discussions with the FDA, with the goal of submitting an NDA in Q4 and securing approval.
Indeed, the market responded positively. Check it out …
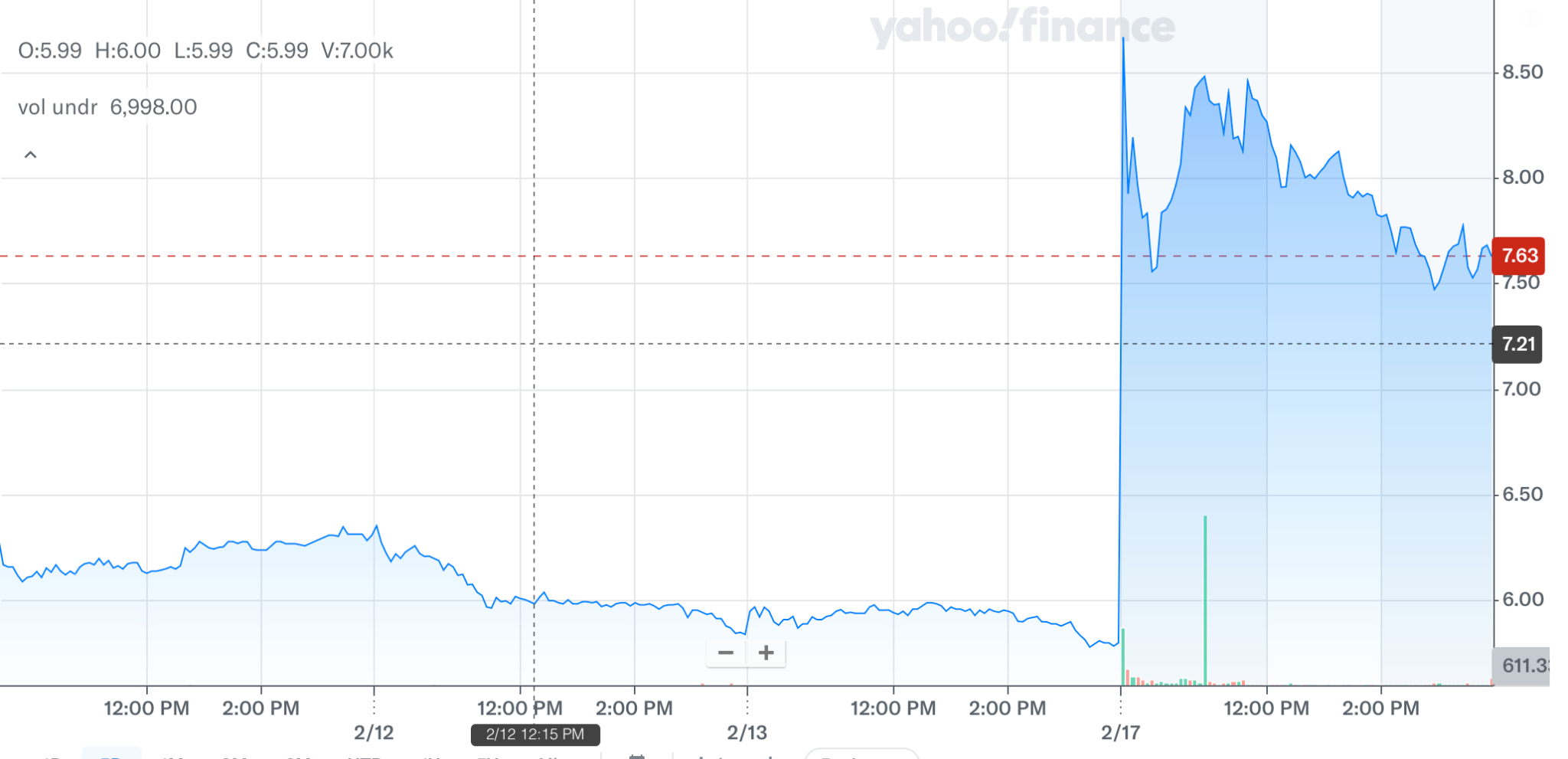
Today’s move is noteworthy because with these positive Phase 3 results, the company’s lead program has been materially de-risked.
Management is already preparing to engage the FDA on a rolling submission, with an NDA filing expected later this year. The clinical profile suggests rapid onset and durability of effect, which is exactly the kind of story regulators and physicians pay attention to.
Of course, positive Phase 3 data doesn’t guarantee smooth stock performance for the rest of the year. But Big Pharma is on the hunt for new TRD treatments, so it wouldn’t surprise me if a bigger pharma player came along and just acquired the treatment outright.
Certainly, this wouldn’t be the first time Big Pharma has shown up with a big check for a psychedelics company.
In fact, last year, AbbVie (NYSE: ABBV) ponied up $1.2 billion to acquire a single investigational therapy for Major Depressive Disorder from Gilgamesh Pharma. That’s huge! So it wouldn’t surprise me at all to see something similar happen with Compass Pathways.
Indeed, we’ll find out soon enough.






.webp)